Directed Evolution Drives Hertz Fellow Kevin Esvelt to Find Solutions to Vector-borne and Parasitic Diseases
The World Health Organization estimates that there were more than 200 million cases of malaria, 250 million cases of schistosomiasis, and perhaps 200 million of dengue in 2015.
Hertz Fellow and evolutionary biologist, Kevin Esvelt, has identified a general way to combat these vector-borne and parasitic scourges by altering entire populations of wild organisms. The technique, called CRISPR (clustered regularly interspaced short palindromic repeats) gene drive, is an example of directing the natural process of evolution so that the resultant changes can benefit humanity for generations to come.
“Evolution created all living things and most useful molecules,” Esvelt said. “Harnessing and directing evolution lets us enact changes that we could never rationally design.”
In December, Esvelt, 33, was appointed as an assistant professor of the MIT Media Lab, where he will lead the new Sculpting Evolution research group to explore ecological and evolutionary engineering. He will be extending the work he conducted at the Wyss Institute and Harvard Medical School, where he helped developed CRISPR in collaboration with his mentor, George Church.
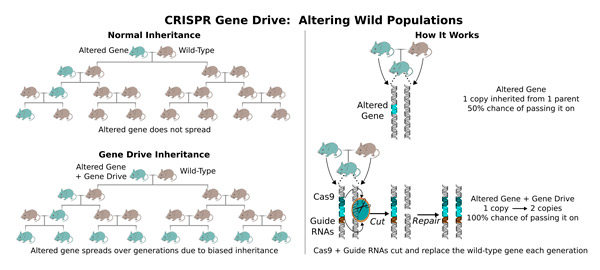
CRISPR, Esvelt said, “amounts to molecular scissors that can be directed to cut – and therefore edit – nearly any gene in any organism.” The technology has revolutionized molecular biology, but initially didn’t change the ability to edit wild populations.
“When we alter an organism, we reduce its ability to survive and reproduce in the wild, causing natural selection to eliminate any changes we make, said Esvelt, who wondered whether encoding the CRISPR editing machinery next to the altered gene could change this.
“If you make an organism with a CRISPR gene drive, nearly all the offspring will inherit the altered gene,” Esvelt said. “It works by making genome editing re-occur in each generation. The original sequence is converted to the edited version, plus the genes encoding the CRISPR components. Using CRISPR gene drive, we can cause most laboratory-made changes to quickly spread in the wild over generations.”
For example, malaria parasites are transmitted through the bites of infected mosquitoes. CRISPR gene drives could be used to spread genes that reduce the ability of the mosquitoes to transmit the parasite. It could be applied in a similar way to immunize the mice that transmit Lyme disease to ticks, which in turn spread it to humans. Lyme is currently contracted by more than 200,000 Americans every year and infection rates are rising steadily. Other potential applications include saving species on the verge of extinction and controlling pests and invasive organisms in an environmentally friendly manner.
“Instead of spraying insecticides that kill both pests and most other insects, we might program the pest population so that they don’t like the taste of our crops,” Esvelt said. “Gene drives offers a completely new way to interact with the living world. We are finally learning to speak using the language of nature rather than with pesticides and bulldozers.”
Esvelt has had an interest in evolutionary biology since his parents took him to the Galapagos Islands at the age of 12, imparting a lasting impression of the majesty and power of evolution. Visiting the place where Charles Darwin conducted his own groundbreaking observation and research on evolution led Esvelt to delve into Darwin’s works, and altered his notions about the role of humans in nature’s course.
“Unimaginable amounts of suffering occur in the wild and evolution doesn’t care,” Esvelt said. “Darwin was horrified by the cruelty of nature. If you care at all about animal suffering, you simply can’t look at the natural world as a place of universal purity and goodness.”
After graduating from Harvey Mudd College in Claremont, California, Esvelt was awarded a Hertz Fellowship in 2004. The prize, along with a grant from the National Science Foundation, gave Esvelt the freedom to pursue his own interests while he earned his PhD in biochemistry from Harvard University. There, working in the laboratory of Professor David Liu, he invented Phage-Assisted Continuous Evolution (PACE), a synthetic microbial ecosystem for rapidly evolving biomolecules. PACE, which Esvelt considers his most innovative achievement to date, was recognized with the Hertz Doctoral Thesis Prize and the Harold M. Weintraub Graduate Student Award.
“PACE is a black box that solves molecular problems. It harnesses a continuous evolution process that doesn’t require human intervention,” Esvelt said. “The rationale is that populations evolve in nature all the time, some of them – such as phages – extremely rapidly. We just need to change the environment so they evolve in ways useful for us.”
While Esvelt sees the potential benefits of RNA-guided CRISPR gene drives, he’s acutely aware of the possible pitfalls. He’s encouraged scientists to make development of the technology a public process and has worked to create and publicize safeguards to make sure gene drive elements can’t accidentally escape the lab and also ensure that any genetic changes can be overwritten if something goes wrong.
“Normally, experiments only affect the people in the lab. When you add a gene drive, the expectation flips: it’s expected to spread throughout ecosystems that others rely on. More, it gives small groups of people the power to unilaterally alter the shared environment. So how are we going to do this responsibly?” Esvelt said. “Keeping it hidden isn’t ideal for this type of technology. We need to handle this differently. It should be transparent so the public can review and weigh in.”
Esvelt and his group at MIT will be employing and refining confinement strategies to safely study gene drive, such as building elements that are only active in lab organisms, working with organisms that can’t reproduce, and conducting research with species that can’t survive in the local environment if they were to escape.
“The primary risk isn’t technical or ecological, it’s social – the issues are public trust, governance, and informed consent. It’s very hard to imagine ecological side effects that would outweigh the devastating impacts of malaria, schistosomiasis, and other diseases we might address using gene drive,” Esvelt said.
So far, research groups have reported CRISPR gene drive elements that are inherited by roughly 95 percent of offspring in malarial mosquitoes, and within a year, Esvelt said, most or all of the laboratory problems should be solved. However, the world is “nowhere near reaching the level of societal discussion and ecological studies” necessary to decide whether and how to test or eventually release these mosquitoes.
“Because gene drive elements will ignore borders, certain international treaties (not signed by the United States) will require the unanimous consent of every country that harbors that species; that’s an issue,” Esvelt said. “We need a new societal approach to address these capabilities now that they’re within our reach. Much more broadly, we must recognize that we don’t decide which technologies to discover; our search is determined by the laws of physics, evolution, and capitalism – and when we find a box, we always open it. Frankly, we often don’t take nearly enough care to open them carefully. I’d love to develop a framework that would help scientists decide how to open these boxes with care and wisdom.”
Esvelt said he hopes gene drive technology can serve as an impetus and test case while avoiding past mistakes.
“We must learn from the missteps that plagued GMO foods. Namely, we should focus on applications with obvious benefits to citizens, initiate discussions prior to experiments, develop the technology transparently, so we can address concerns during the design-build-test cycle, invite community guidance of safety testing, do it all in the nonprofit sphere, and clearly acknowledge that we won’t proceed without public support.”
At MIT, he intends to continue pursuing gene drive applications and safeguards while creating new models for responsive and community-guided science. He’ll also explore other fitness-positive technologies that could engineer microbial ecosystems to better protect against pathogens, explore better ways to evolve molecular tools, and seek to remedy the most concerning faults of natural evolution by reducing animal suffering.
“It’ll be a tremendous amount of fun and will challenge me in a lot ways,” Esvelt said. “And because I’ll be at the Media Lab, we’ll be able to pursue questions that are interesting to me and many others that would be hard to explore in a more traditional department.”